Fluorescent lamps and ultraviolet radiation
Olive oil
What is the relationship between fluorescent lamps and ultraviolet radiation?
We will attempt to put in simple terms what goes on inside a fluorescent lamp. As you may note in the diagram below, a fluorescent lamp is made up of many components.
When a fluorescent lamp is turned on, electrons begin to travel at high speed from one cathode to the other, establishing an electric discharge or arc through the mercury vapor. An arc of this nature, enclosed in a glass tube with internal gas pressure, produces ultraviolet energy.
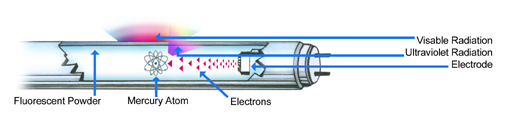
This ultraviolet energy is converted into visible light by the phosphors, which have the ability to absorb the ultraviolet energy and re-radiate it at longer wavelengths that can be seen as visible light. The color of the visible light produced depends on the chemical composition of the phosphor coating on the inside of the glass tube.
Approximately 60 percent of the input energy in a typical 40 watt fluorescent lamp is converted directly into ultraviolet, with 38 percent going into heat and 2 percent into visible light. Standard phosphor changes about 21 percent of the ultraviolet into visible light with the remaining 39 percent converted to heat.
The final output of convected and conducted heat from typical fluorescent lamps is very damaging to sensitive perishable fresh foods. It causes the surface of many products to fade and discolor, while also warming the merchandise, resulting in moisture evaporation and drying.
This exposure is not considered harmful to people in office, school or general lighting situations.
